Introduction: Pediatric sepsis is a major life threat due to multiple organ failure (MOF) progression and resistance to conventional therapies. Extracorporeal hemoperfusion (EH) using Efferon LPS adsorber abrogates MOF and halts septic shock (SS) in adult patients [1]. Here, we report case series of children with sepsis to assess the feasibility and safety of EH with a new pediatric Efferon LPS NEO device (PE).
Methods: PE contains porous polymeric scaffold with the surface-immobilized LPS-selective ligand. Seventeen children (9 girls) with sepsis (10 with SS), average age and range: 26 (1–159 months), were included in the study following Ethical Committee approval. Children received two treatments, lasted ≥ 4 h, 24 h apart, with PE. The blood cell count, levels of C-reactive protein (CRP), procalcitonin (PCT), creatinine, urea, ALT and AST were monitored on days 0, 1, 2, 3, 5, 7 and 14. Plasma IL-1b, IL-6 and TNF were determined on days 0 and 3 after the inclusion into the study. STATA 16.0 (StataCorp, USA) were used to assess significance.
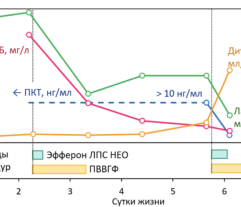
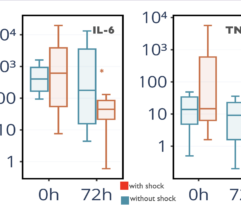
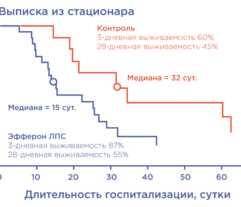
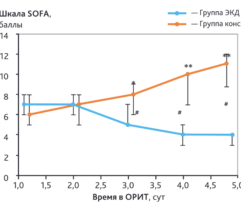
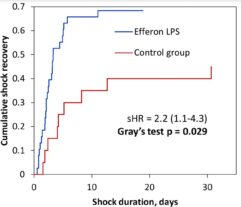
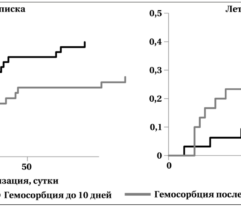
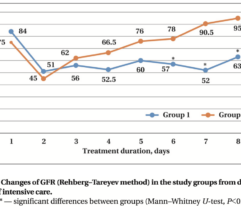
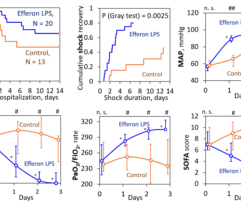
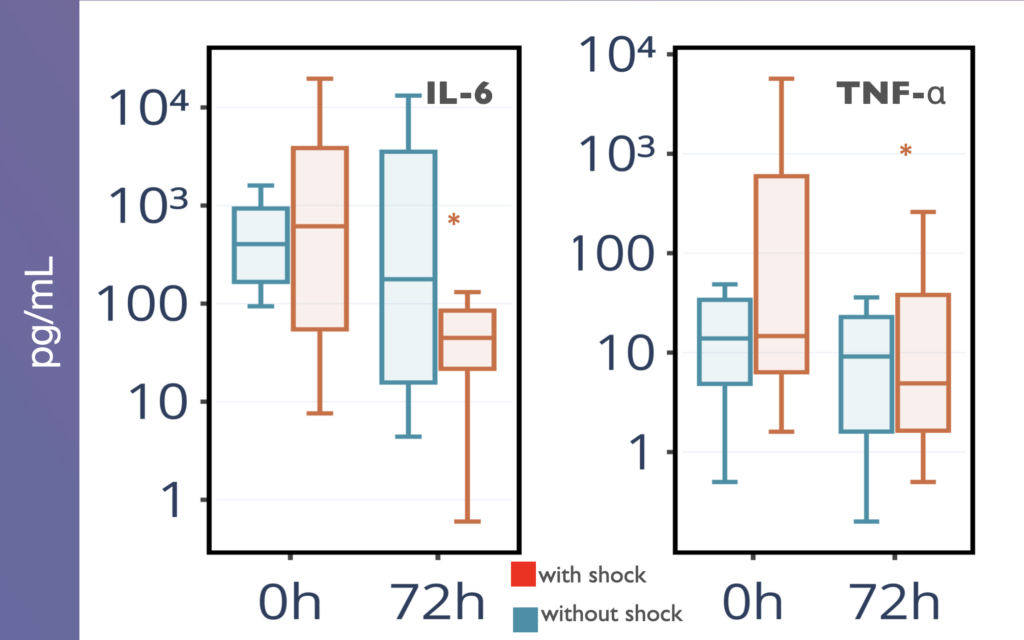
Results: In SS patients, EH resulted in: (a) early abrogation of shock as confirmed by a decrease in vasopressors dose (Figure panel D), (b) MOF reversal as determined by pSOFA (p = 0.003, Figure panel A), (c) lower creatinine (p = 0.006, Figure panel C) and C-reactive protein levels starting from days 2–3, (d) increases in PaO2/FiO2 index on days 7 and 14 (p = 0.004, Figure panel B). Children exhibited significantly decreased cytokines levels concentration in 72 h post-EH (Figure panels F and G). In non-SS children, the cell ratio-based biomarkers exhibited early vs late prognosis for SII and NLR (Figure panels I and H, respectively). Decreased thrombocyte levels on days 1–3 post-EH were normalized on day 7. Only 2 of 17 most severely ill children with refractory SS have deceased by the 60 day.
Conclusions: These results argue for feasibility and safety of EH with PE in pediatric sepsis.