Extracorporeal hemoperfusion (EHP) may improve the course and outcomes of patients with septic shock by targeting cytokines or bacterial endotoxins (lipopolysaccharide [LPS]). Here, we present the results of a multicenter randomized controlled trial (clinicaltrials.gov/ct2/show/NCT04827407) to assess the efficiency and safety of Efferon LPS hemoperfusion cartridges engineered for multimodal targeting LPS, host-derived cytokine and damage-associated molecule pattern molecules.
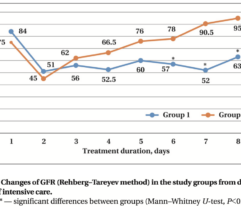
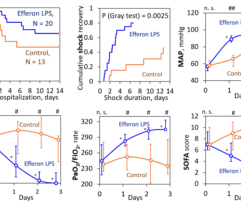
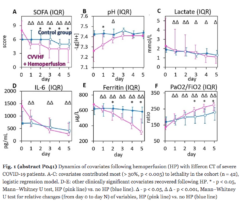
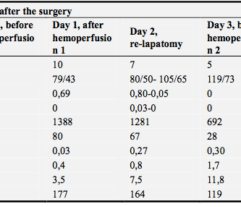
Patients with intra-abdominal sepsis (IAS) and septic shock (Sepsis-3) were subjected to EHP procedures (n = 38). Control patients with IAS and septic shock (n = 20) were treated using conventional protocols without EHP. The primary endpoint was resolution of septic shock. Secondary endpoints included MAP, vasopressor drug dose, PaO2/FiO2 ratio, SOFA score, length of stay in the ICU, and satisfaction with device use by a 5-point Likert scale. Clinical laboratory tests for a blood cells count, lactate and creatinine concentration, nephelometry test for C-reactive protein, immunochemiluminescent test for procalcitonin and immunoenzyme analysis for IL-6 concentration were used to monitor the EHP effect vs. the control group.
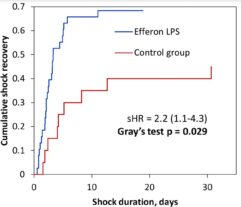
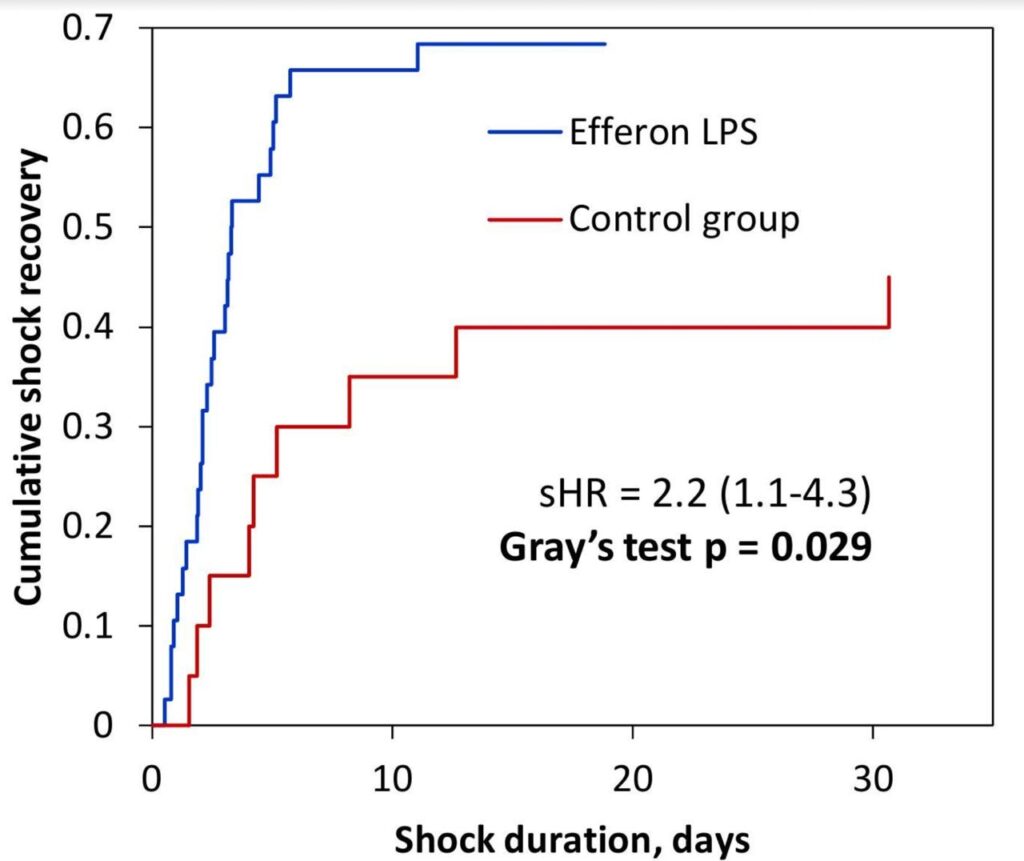
Data were analyzed followed the intention-to-treat approach. Wilcoxon STATA 16.0 (StataCorp, USA) and Excel 2019 with XLStat 2019 add-in (Addinsoft) were used for statistical analysis of the results. The Fine and Gray method of competing risks was used to analyze the primary endpoint and other data representing the time to event.
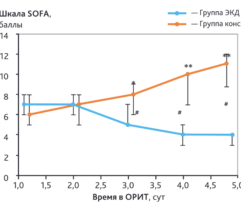
EHP resulted in a significant and rapid increase in mean arterial pressure (MAP) and PaO2/FiO2 ratio, progressive decline in norepinephrine doses, and multiorgan deficiency, as evaluated by SOFA scores. Importantly, EHP led to significantly rapid cumulative mechanical ventilation weaning compared to control group (sHR = 2,5; р = 0.037). Early 3-day mortality was significantly reduced in the Efferon LPS vs. control group, however, no significant improvements in survival in 14 and 28 days was revealed. Laboratory tests showed rapidly decreased levels of LPS, procalcitonin, C-reactive protein, interleukin-6, creatinine, leukocytes, and neutrophils only in the Efferon LPS group. Results demonstrate that EHP with Efferon LPS is a safe procedure to abrogate septic shock and normalize clinical and pathogenically relevant biomarkers in patients with IAS.