Introduction: Direct extracorporeal removal of inflammatory mediators with various adsorbents has been suggested as a novel treatment modality for COVID-19 patients [1]. Our study determined safety, feasibility and effectiveness of clinical use of a hemoperfusion (HP) with a novel SDC adsorber to remove pro-inflammatory molecules from the bloodstream of COVID-19 patients.
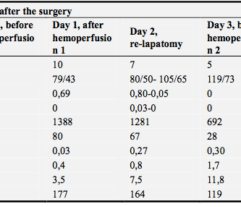
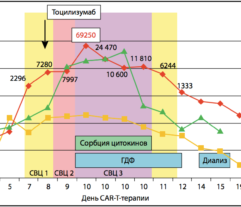
Methods: A total of 42 COVID-19, PCR + patients, age < 81 years, average 63 (SD range, 54–69) years, 52% men, lethality 48%, underwent standard treatment for COVID-19. On day 0, patients exhibited (median and IQR): PaO2/FiO2 160 (130–200); APACHE II 18 (18–20); SOFA 6.5 (6–8); IL-6 841 (709–1424) pg/ml, CRP 109 (84–140) mg/l; ferritin 628 (547–678) µg/l. Group 1 (n = 29) continued to receive standard treatment whereas group 2 (n = 13) received HP procedure once, for 3–4 h, using Efferon CT adsorbers containing mesoporous SDC beads uptaking 6–60 kD molecules [2, 3] followed by continuous veno-venous hemodiafiltration. Groups matched on age and sex; group 2 included more severe patients requiring HP support. Data were treated using R-statistics by XLStat.
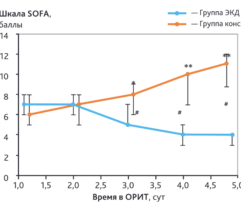
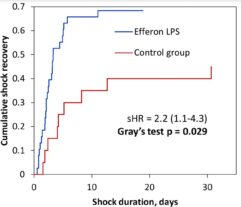
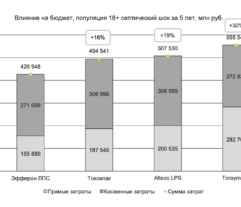
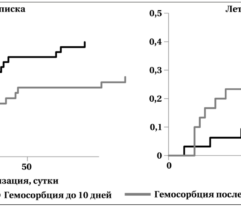
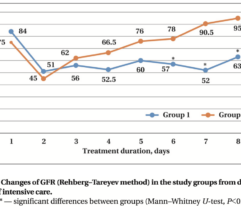
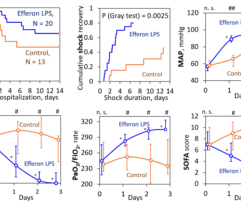
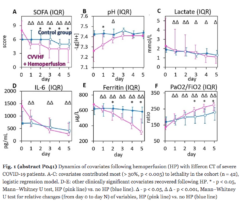
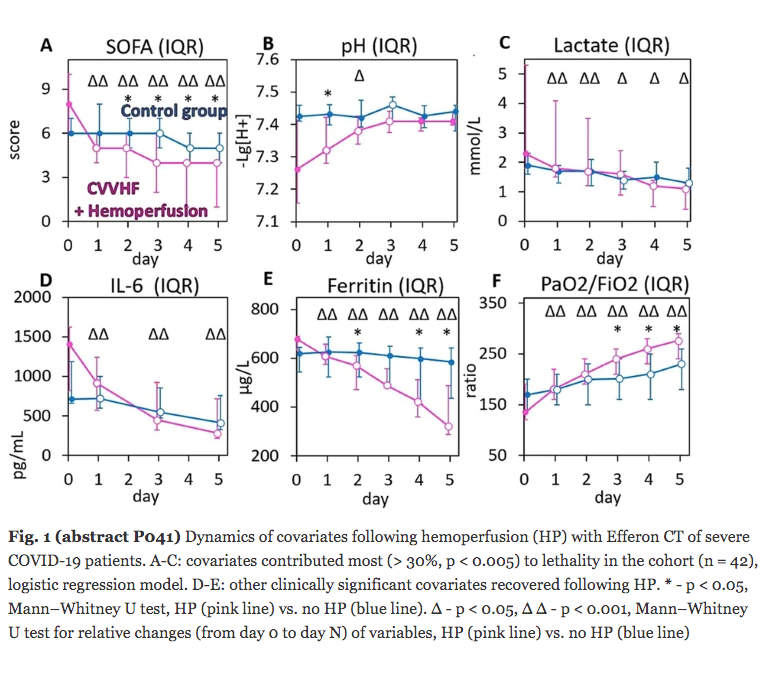
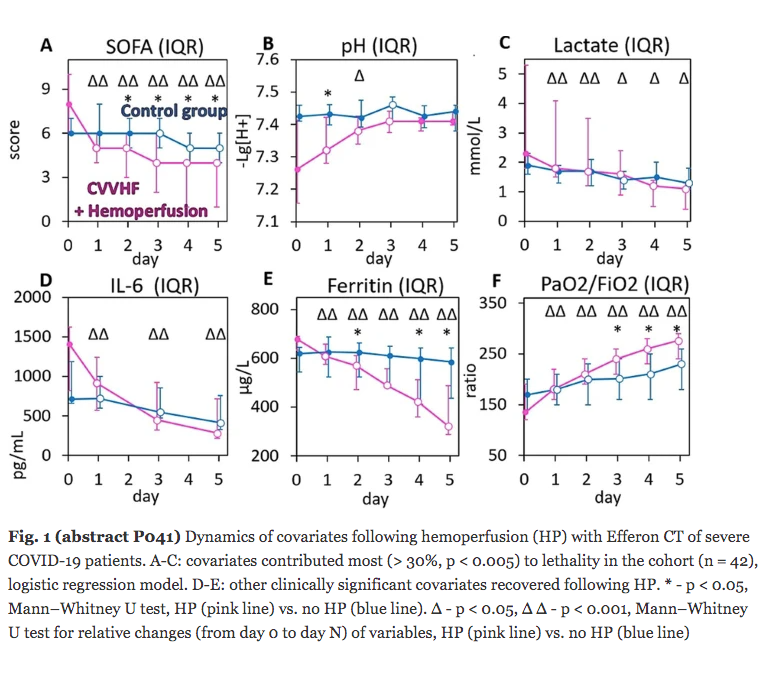
Results: Despite significant intergroup differences in disease severity as shown by SOFA, ALT, AST, SpO2, pH, lactate, LDH, and IL-6 alterations on day 0, the HP treatment resulted in no difference in lethality between groups. Patients on HP experienced pH increase and rapid decline of SOFA and lactate as covariates most contributed to lethal outcome; IL-6 and ferritin levels significantly decreased vs. group 1 (Fig. 1). Decreased lethality by HP was significant in a subset of patients on mechanical ventilation exhibiting SOFA < 9 (p = 0.024 vs. group 1).
Conclusions: Anti-cytokine HP with Efferon CT adsorbers is feasible and safe method providing a lifesaving promise for a subset of patients with COVID-19 that warrants extended clinical trials.